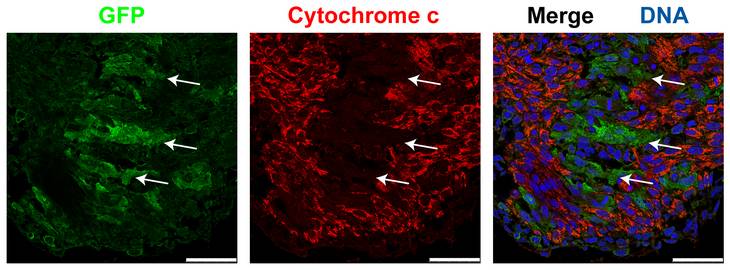
The postnatal mammalian heart is considered a terminally differentiated organ with little (if any) regenerative capacity. Consequently, the loss of cardiomyocytes due to various pathological stimuli (such as ischemia, toxins, drugs, viral infections or genetic alterations) inevitably results in reduced cardiac function and ultimately heart disease or failure. In contrast, the prenatal heart possesses an impressive growth plasticity in response to both endogenous as well as environmental or maternal conditions. Our recent findings have shown that the embryonic murine heart has a remarkable regenerative capacity. We have inactivated the X-linked gene encoding Holocytochrome c synthase (Hccs), an enzyme essential for normal function of the mitochondrial electron transport chain, specifically in the developing mouse heart. Loss of HCCS activity results in mitochondrial dysfunction causing disturbed cardiomyocyte differentiation and proliferation. In contrast to the observed mid-gestational lethality of hemizygous Hccs knockout (KO) males, heterozygous females appeared normal during postnatal life with surprisingly few clusters of defective myocardium, considering an expected mosaic of affected and normal cardiomyocytes as a result of random X chromosomal inactivation. However, analyses of heart conditional heterozygous Hccs KO female (cHccs+/-) embryos revealed the expected 50:50 ratio of HCCS deficient to normal cardiac cells at mid-gestation with a progressive reduction in disease tissue to 10% prior to birth. We could show that this significant change is accounted for by increased proliferation of remaining healthy cardiac cells. These data reveal a previously unrecognized but impressive regenerative capacity of the mid-gestational heart that can compensate for an effective loss of at least 50% of cardiac tissue to enable formation of a functional heart at birth.
Ongoing projects aim to identify the molecular mechanisms that drive regenerationof the embryonic heart in cHccs+/- females, including both induction of proliferation in healthycells as well as the role of diseased (HCCS deficient) cells.We have recently performed RNA expression profiling on whole embryonic hearts using microarray technology and our current research focuses on the molecular signature of the healthy cardiomyocyte population in the embryonic cHccs+/- heart. These studies revealed a multitude of interesting candidate genes and pathways involved in heart development, cell proliferation, cardiac growth and metabolism which appear to be induced in these cells. The most promising pathways and genes emerging from these analyses will be functionally evaluated for their potential to induce cardiomyocyte proliferation in vitro as well as in vivo. If applicable, pharmacological or genetic inhibition of the candidate pathways will be applied to directly prove their functional relevance for embryonic heart regeneration. Finally, our previous results suggested that defective (HCCS deficient) cells might take an active part during embryonic heart regeneration by secreting certain growth factors or cytokines which in turn induce proliferation in the neighbouring healthy cells. So another goal of our current work is to seek and characterize such paracrine proliferation signals using proteome and secretome analyses in embryonic cHccs+/- compared to control hearts. Taken together, the investigation of growth factors and pathways mediating compensatory proliferation of healthy cardiomyocytes during embryonic heart regeneration is of great interest for various aspects of normal and impaired heart development as well as for the identification of possible therapeutic targets for future regenerative therapies in the postnatal heart.