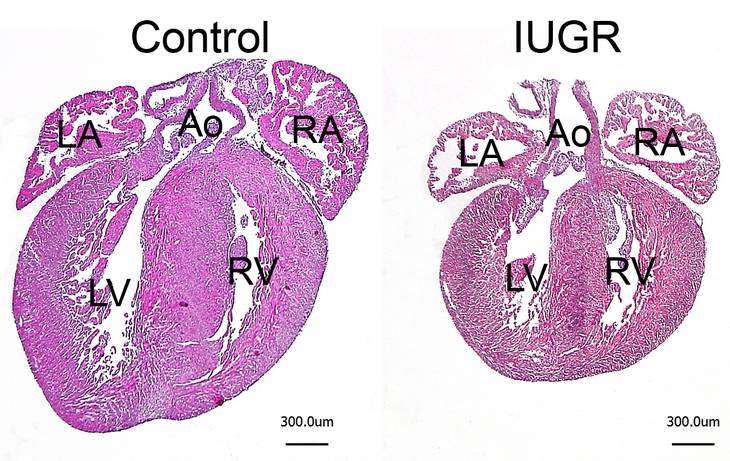
Unfavorable intrauterine growth conditions have beenshown to predispose the heart for cardiac disease later inlife, a process referred to as fetal programming. Generally, intrauterine growth restriction (IUGR) often results in reduced heart size at birth characterized by a reduced complement of cardiomyocytes. We have recently shown in mice that neonatal cardiac hypoplasia alters postnatal cardiac growth pattern, resulting in transient normalization of organ size until early adulthood by accelerated cardiomyocyte hypertrophy (i.e. increase in cell size). This compensatory hypertrophy cannot be maintained throughout life time, however, such that heart size is reduced again upon ageing. Neonatal hypoplasia also alters cardiac stress response in adulthood, characterized by an overshooting hypertrophic growth and aberrant molecular growth signaling. Inhibition of the latter results in contractile dysfunction in developmentally impaired hearts upon stress. This clearly confirmed that disturbingprenatal cardiac development renders the response ofthe postnatal heart to pathological conditions. Based on these data we propose a new model linking fetal programming to growth and organ size control in the adult and ageing heart.
We are currently investigating the consequences of different IUGR models for growth of the pre- and postnatal heart as well as cardiac stress response in adulthood. A special focus lies on mTOR, an important regulator of translation and cell growth. Whereas mTOR has been intensively studied in the context of pathological as well as physiological cardiac hypertrophy in adulthood, surprisingly little is known about its relevance for the prenatal heart. Therefore, we developed a rapamycin treatment protocol in pregnant mice to efficiently induce IUGR in the offspring and study the importance of mTOR for perinatal cardiac growth. Current projects intent to further study its role during embryonic and fetal heart development using genetic models of heart conditional inactivation of mTOR components in mice.
IUGR in humans is often caused by impaired nutrient supply to the embryo or fetus, which can be a result of maternal undernutrition or placental insufficiency (among others). In animal models IUGR can be induced by protein restriction in the maternal diet during pregnancy, which in the offspring results in cardiac hypoplasia at birth due to a reduced number of cardiomyocytes. To assess the importance of amino acid availability for cardiac growth and organ size control, we are investigating the consequences of a low protein diet throughout pregnancy in mice and rats. Our preliminary results suggest that amino acid restriction does not alter developmental patterning or growth of the embryonic heart, but results in reduced heart size in adult mice if kept on a low protein diet after birth. Thus, sufficient amino acid availability appears to be primarily important for postnatal hypertrophic but not prenatal proliferative growth of the heart. Current projects intend tothoroughly characterize the role of amino acid availability during the transition from pre- to postnatal cardiac growth pattern.